- India
- International
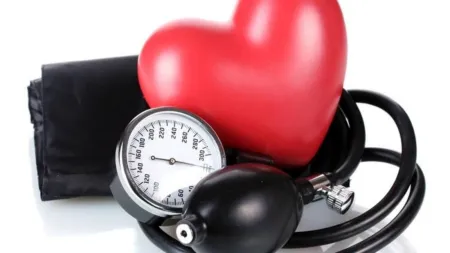
Medical devices are not drugs
Why government should bring a new law to regulate medical devices, not apply the 80-year-old Drugs and Cosmetic Act
 This attempt to deliberately retrofit medical devices into the Drugs and Cosmetics Act will lead to a toothless regulatory framework
This attempt to deliberately retrofit medical devices into the Drugs and Cosmetics Act will lead to a toothless regulatory framework
Astonishing things are happening in the Ministry of Health and Family Welfare these days. In less than 30 days, the ministry is going to put in place a regulatory framework for medical devices that will likely give lobbyists for the medical device industry a wonderful Christmas present.
Amazingly enough, the ministry aims to implement this new framework without the Parliament casting a single vote to determine the future of an industry which has already proven itself to be brazenly irresponsible towards patients in India as we are seeing in the ongoing hip-implant scandal.
Rather than moving a new law in Parliament to regulate the medical device industry, the ministry is creating an entire regulatory framework out of notifications and rules, using powers delegated to it under the Drugs and Cosmetics Act, 1940 — a pre-independence legislation that was enacted almost 80 years ago.
The first move towards the new framework was made in 2017 when the ministry, using powers under the Drugs and Cosmetics Act, notified the Medical Device Rules, 2017. At that time, only a few medical devices were notified as “drugs”. In a notification a few days ago, the government announced its intention to treat all medical devices, be it an implant or a MRI machine as “drugs” under the Drugs and Cosmetics Act.
This means that all medical devices would be placed within the framework of the Medical Device Rules, 2017. This attempt to deliberately retrofit medical devices into the Drugs and Cosmetics Act will lead to a toothless regulatory framework for devices, similar to what exists for drugs today. I say this for two reasons after having spoken to legal experts.

First, the ministry cannot create new offences or penalties through its rule-making authority. Only Parliament can enact a law that creates new offences and penalties for wrongdoing. As a result, the Medical Device Rules 2017 contain no penal provisions. Although the Drugs and Cosmetics Act does contain a penal provision for the manufacture of sub-standard drugs, it cannot be used to penalise manufacturers of sub-standard medical devices (although medical devices will now be legally defined as drugs) because legally binding standards recognised in the Second Schedule to the Drugs and Cosmetics Act covers only pharmacopeias for drugs. If no standards for medical devices are recognised in the Second Schedule, it follows that there can never be a prosecution of a manufacturer of sub-standard medical devices.
What will unfold, if the current proposal is adopted, will be similar to what we see with substandard drugs: Companies that make defective products will recall them from foreign markets while continuing to sell the same product in India, and comply with the law. There is one subtle distinction here.
Even though sale of sub-standard drugs can be prosecuted under the current law; because there is no political will, most manufacturers who make poor quality drugs go scot-free. Under the present proposal, even if there is public pressure, similar to what we have seen in the faulty hip-implant case, no prosecutions will take place because there will be no basis to prosecute intentional wrongdoing in the law.
More importantly, medical devices, because of their nature, will be far more difficult (if not impossible) to standardise when compared to drugs. So, what are the tools available to Indian regulators under the proposed framework to hold makers of sub-standard medical device manufacturers to account? The answer is none whatsoever. At most, the ministry can prohibit the manufacture and sale of certain medical devices under Section 26A or cancel a license to prevent future harm. But, what of penalties or prosecution to punish for the harm already inflicted on patients due to negligence or worse, intentional wrongdoing by the manufacturer?
The second reason is that it is quite clear that the ministry has not learnt any lesson from the recent hip-implant debacle. One of the main challenges faced by the government in securing justice for faulty hip-implants was to secure a list of patients who had received the implant through surgery.
Since the manufacturer sold these devices to doctors and hospitals and not patients directly, it did not have a list of patients who had these devices implanted. It could therefore conveniently skirt the government’s query for a list of all the patients with faulty implants. Neither the doctors nor the hospitals have an incentive to share the list of patients or even inform the patients because it would mean opening themselves up to legal liability for surgically implanting faulty devices in the patient’s body. One of the solutions to solve this information deficit is to create a confidential patient register that should be maintained by the government to record all details of implants.
This register could be used to notify patients in the case of malfunctioning devices. The proposed regulatory framework does not offer any such provision. As a result, the next time there is such a case, the government will once again not be able to contact the patients to inform of their rights.
While medical devices have tremendous potential to revolutionise healthcare, the hip-implant scandals in India and abroad have shown us the dark underbelly of this industry. The proposed regulatory framework lacks teeth and will do little to hold the powerful medical devices industry accountable in cases of intentional wrongdoing. The government must rethink this toothless framework and instead enact a new law through Parliament.
Medical devices are not drugs, and it would be a grave mistake to apply the same regulatory framework to regulate these complex devices. The need of the hour is for new ideas to regulate this industry in the Indian context where the courts lack the capacity to tackle such complex issues.
The writer is a public health activist who blew the whistle on Ranbaxy Laboratories
EXPRESS OPINION
More Explained
Apr 19: Latest News
- 01
- 02
- 03
- 04
- 05